CLINICAL TRIAL READINESS ASSESSMENT

Are you really ready to run a clinical trial? How can you best assess your clinical trial readiness? We work with C-level executives and management boards and understand their objectives, constraints and timelines. Do this before buying services for clinical data management, EDC, ePRO and biostatistics. Using a fixed price, fixed delivery model, our clinical trial readiness […]
Dealing with information junkies in decentralized clinical trials

DecentraIized clinical trials software vendor Medable is doing an outstanding job in understanding and executing an online work-flow between sites and patients at home.. This understanding and execution is a necessary but insufficient condition for the success of decentraIized clinical trials. At their core, decentraIized clinical trials and hybrid studies have the same issues as any clinical research project. These […]
How to meet the 10 top challenges in Phase 1 clinical trials

Phase 1 challenges are unlike larger Phase 2, Phase 3 studies. The science is still unsure. The the clinical operations team at a startup may still be under construction. In this post, I’ll share our experiences at flaskdata.io with early stage drug and device vendors doing their first Phase 1 safety study. You’ll see a […]
SDV sucks
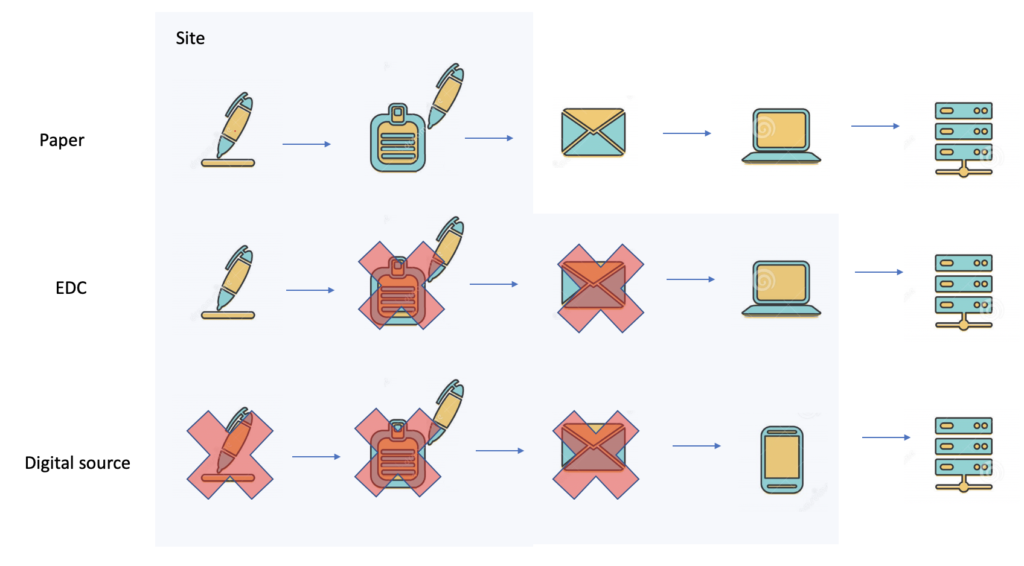
How professional services delay cures for patients. Why SDV Sucks In the early 50s of the previous century, nobody knew that 70 years later clinical trials could be run using a phone. Clinical trials that were performed 70 years ago used paper forms. By the late 70s, batch-driven data processing systems were introduced. Paper forms […]
Clinical data management, payment for outcomes

Payment for outcomes using GCP metrics is a way to significantly reduce clinical operations costs and increase value-for-money from your CRO or DCT vendor. Payment for outcomes using GCP metrics shifts study costs from billable activities to measurable results. Professional service activities are hard to control. GCP metrics are hard to contest. In this post, […]
Why you do not want to unify data in your clinical trial

Unified clinical data sets – good or bad? Unification of data from patient medical records, hospital reports and clinical trial protocols is a tempting yet extremely dangerous idea. In this outstanding guest post, security and privacy expert, Veronika Valdova from Arete-Zoe explains why merging medical records, hospital reports, and clinical trial data is a very bad idea. How data breaches […]
Career development for clinical data managers

A good clinical data manager is an essential piece of running a clinical trial. A well-trained and responsibility clinical data manager is a an important part in study execution. A good clinical data manager will want to have a career development plan. A clinical data manager on an individual contributor track, needs to develop […]
Did you wait until the last minute to choose vendors for your clinical trial?

Your dream outcome. Helping cancer patients. Doing it right. Recruiting quickly and efficiently and getting a diverse population into your study. You have noble intentions and great science. So – why are you accumulating months delay even before the first patient is recruited? There are a lot of things on the science and recruitment side, […]
Data capture by sites is activity, not achievement

Never mistake data collection activity for an achievement Recruiting and caring for patients (whether at home or on site) is a research site responsibility. Capturing pages (note the paper paradigm!) is one of the key billable metrics for a site. While data collection, detection of exceptions and action to close issues are all activities essential […]
Patient compliance in clinical trials – the elephant in the room

Clinical trials cost a fortune. When a patient is non-compliant during the run-in/washout period, he/she is dropped out of the study. This costs the sponsor 5X: site costs for 2 patients, recruitment costs for 2 patients and loss of time. In other words, retention is more important than recruitment. This raises the question: why is […]
