The clinical data management plan, starts with developing a robust data model according to the protocol.
The data model is designed to efficiently serve GCP monitoring of the study and fit the needs of the statistical analysis of the results. The data model design is a team activity led by a Flask Data project manager with you and the study statistician. The output of the data model design is a specification which is version controlled and updated based on clarifications and/or requests received from the customer. The data model specification also serves as a basis for quality control of the study build.
The team then decides what real-time data tools they need for clinical data and safety monitoring, patient compliance monitoring and site performance to service level objectives for data collection.
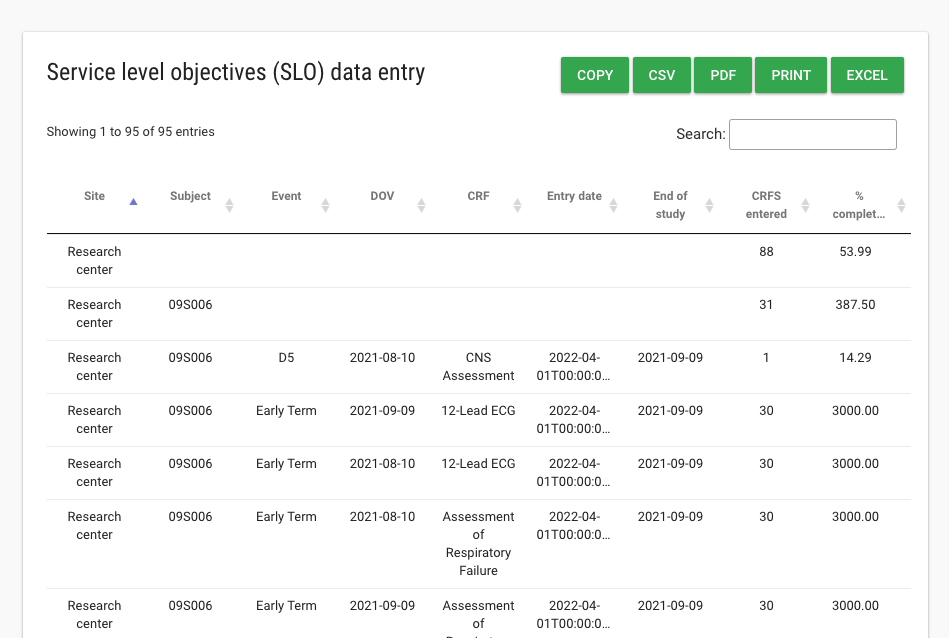
The process of continuous data clean brings the study team to objective of study: testing and validating the scientific hypothesis of the experiment. Flask Data provides validated CDISC-standard data extract formats including ODM XML 1.3, CSV and SDTM/ADaM data set preparation.

Clinical data made radically simple and affordable.
Copyright © 2022 Flask Data Ltd. All Rights Reserved.