First mover advantage for clinical trials
Although the notion of same day delivery seems to be recent, in fact, competitive advantage always went to those who can move quicker. Even if they are not the first. With fast communications, there is now a relentless trend towards increasing speed. We witness this trend in almost every industry:
- Retailers – Intercepting abandoned online shopping carts could recover 2.75 trillion USD
- Autonomous vehicles – A disruptive product only possible through real time data processing
- Financial industry – Bloomberg terminal, Fraud detection, automated trading, real time payments
- Communication – making written communication real-time via online messengers
- Military, Government, Construction, Real Estate, Healthcare – all these industries are affected.
The first and last miles in clinical trials
The first mile is raw data.
EDC created huge efficiencies and increased the speed of data flow from sites to databases. However, the C in EDC is misplaced. Modern EDC systems do not capture data. They are eCRFs, not EDCs. They just move data entry to sites, forcing hospital staff to become data entry clerks. The key issue – separation of data capture and data entry – still remains.
We can picture the differences between paper, eCRF/EDC and all digital solution like this:
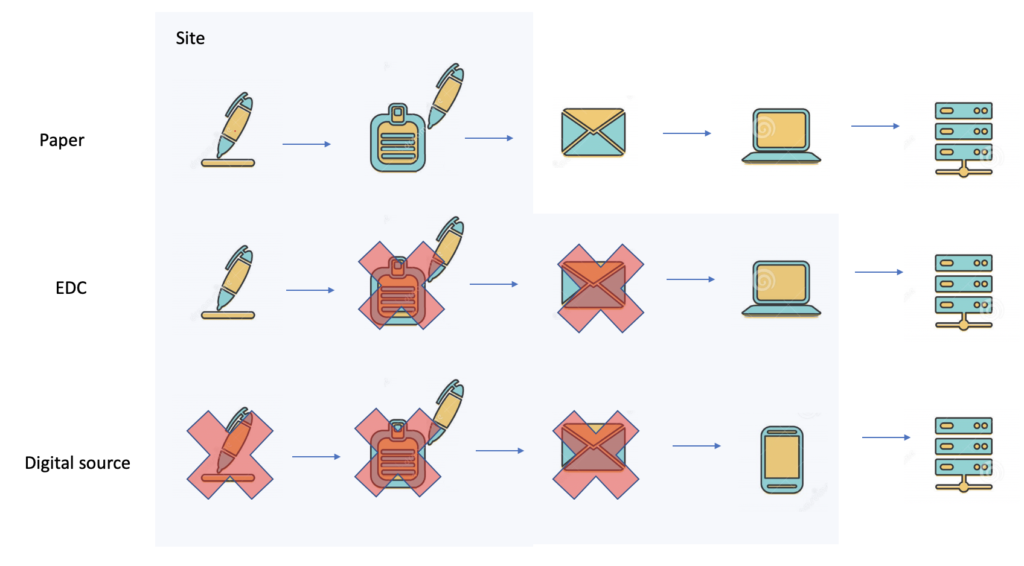
Removing the extra step of data capture speeds clinical data acquisition by up by several weeks. Currently, contracts with sites, stipulate data entry cycles of up to 5 days. This can be reduced by a combination of DDC, ePRO, eCOA and ingestion of medical IoT and lab data without achieving the holy grail of EMR integration.
In fact – decentralized clinical trials do very little to reduce site workload and arguably in many cases increase site workload due to introduction of more systems to the site by sponsors.
An all digital solution for all clinical trials is utopia. Although, there are many interesting use cases and business models for all-digital studies; the hard-core drug trials in oncology still rely on hospital sites. Integrating EDC with hospital EMR is a complex problem with expensive solutions, fraught with turf politics, security and privacy issues, data interface challenges, data mapping, and system interface problems. Cloud platforms that interface EHR systems, like Redox Engine are leading the way in clinical care but are still stymied in clinical trials.
The last mile is data integrity and patient safety monitoring.
There are in fact 2 blockers in clinical trials – data acquisition and data and safety monitoring. Data and safety monitoring is typically 15-30 days behind schedule, due to manual processes of data management and reporting. If we accept the site pain and first mile data acquisition latency due to manual transcription of 3-5 days – it seems that we can vastly improve our ability to assure data integrity and patient safety by automating the monitoring portion of the process.
Let’s assume that for the foreseeable future, we will have to continue to live with multiple sources of data in different media, formats and access methods.
This is a reality of web1, web2, and web3 – multiple diverse data sources, but interoperable.
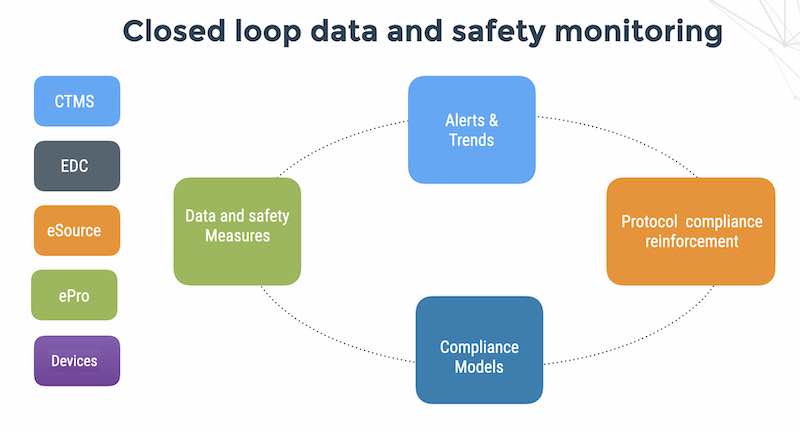
In fact, SDTM, XML and FHIR are not outstanding formats for fast interoperability. More likely candidates would be JSON messages on a queue or protocol buffers. The discussion on interoperability will have to wait for another post.
In summary:
Fast data is important but it is not the last mile of clinical trials.
The last mile of clinical trials is data quality and patient safety monitoring.






