Thoughts on monitoring patient data and safety in clinical trials

The future: tech-driven data and safety monitoring
Running clinical trials with the help of tech is becoming much more prevalent, but it’s unclear if data and safety monitoring has kept up.
In this essay, I discuss how patient data and safety monitoring fell behind the pace of digital transformation of clinical trials. I will present a tech-driven approach for monitoring and reducing risk to patients in clinical trials.
COVID-19 accelerated use of digital tech in clinical trials
During COVID-19 (which now seems to be the new normal), drug companies started running so-called decentralized clinical trials (DCT) with the help of digital technologies like Zoom and mobile apps. Until now, pharma went to hospital systems and specialty research sites to recruit patients. With COVID-19 — that option became almost impossible.
Use of decentralized clinical trials is now growing quickly. By automating and streamlining the clinical trial process, many of the expensive and time-consuming tasks (like transcribing paper to computer) in traditional drug trials can be eliminated.
This helps bring new drugs and vaccines faster to patients.
It also creates new risks to patient data and safety.
The basics of technology risk
- Risk does not come out of thin air. You need to monitor and analyze risk.
Suppose you’re the VP Sales of a chain of pharmacies. You might guess that you’re losing customers because of parking and public transportation. By analyzing parking capacity and bus stop locations, you can create an accessibility metric and measure the risk to losing business because the pharmacy locations are not accessible to people. - Software products often promote new features (like being faster) without considering security.
- The world of tech is divided into camps. Application camps, database camps, cloud camps, AI camps and many more.
- People in the application software camp, almost never talk about the role of their software in data and safety monitoring. It’s almost always someone else’s problem.
- Application software is notoriously vulnerable. Buggy software is vulnerable software.
- The world is complex. Clinical trials have software running on patient phones, site coordinator tablets, on hospital systems and FedEx APIs. More complex means more things will go wrong.
- The world is is moving faster than the government. The latest FDA guidance for monitoring clinical trials was released in 2013. In other words, don’t expect regulation to effectively mitigate tech risk.
How patient data and safety monitoring fell behind
Clinical trial software companies typically tout the advantages of DCT for faster recruitment and availability to more diverse patient populations.
Unfortunately, buzzwords do not automatically solve all our problems.
There are many activities in a clinical trial but only 2 count:
Two factors determine risk in a clinical trial: patient recruitment and data integrity and patient safety monitoring.
Recruitment is an activity that impacts the beginning of patient journeys in a clinical trial. More patients is like more water flowing into a pipe.
Data and patient safety issues impact the risk of the patient journey throughout the clinical trial. Data and safety issues are like leaks in the piping; and it’s very hard to find leaks.
When you have data integrity and patient safety issues, using pre-screening and deploying a network of 1,000 pharmacies for recruiting people is not going to help. It just makes monitoring that much harder — like finding leaks.
The challenges of data and safety monitoring in DCT
DCT tech is 2021. The methods of data and safety monitoring are 2013.
The volume, variety and velocity of data in a modern decentralized trial is 5–10X a traditional site-centric trial. This is because we now have patients, mobile apps, wearable devices and supply-chain partners all contributing data as well as the traditional 5–7 applications used by research sites.
If you have 20,000 patients and 50 metrics — that’s 1M metrics to track on a timeline of slow-moving, unordered events. We could limit metrics to patients — but then we’d miss risk from hardware and software bugs in apps and connected devices. We’d also miss anomalies with investigators.
By the time you see data, safety and patient compliance issues in a DCT dashboard, it’s too late to fix. The data is flowing too fast.
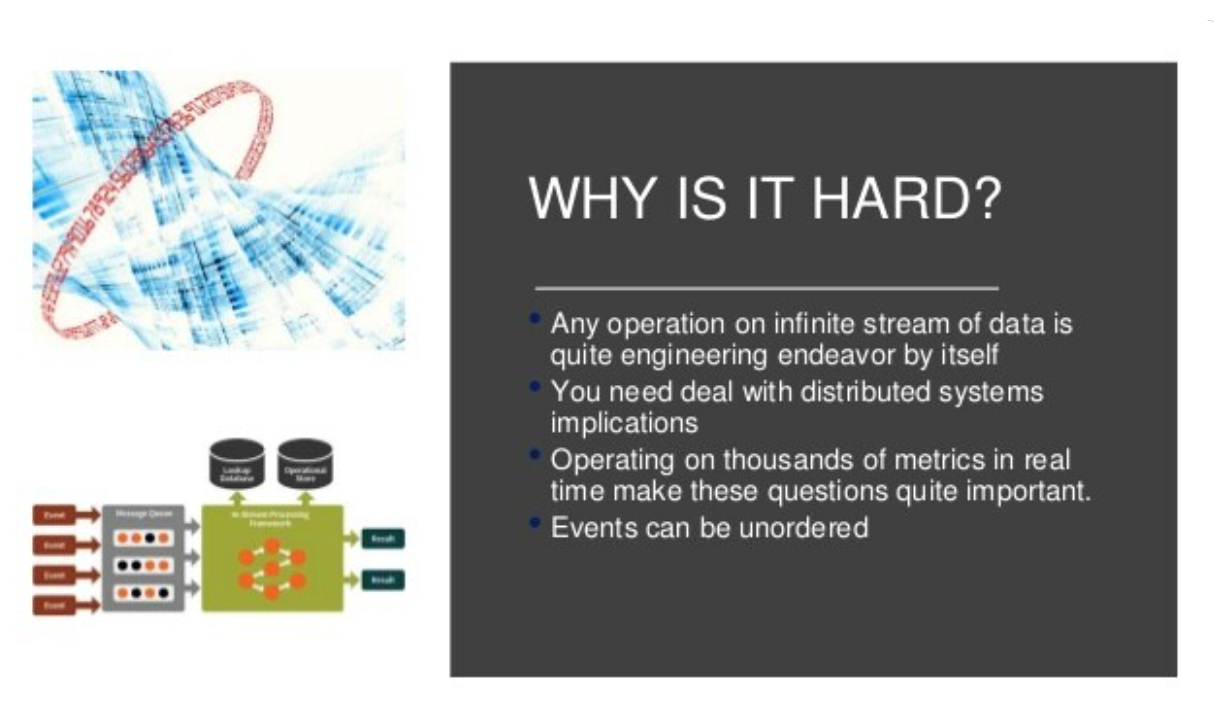
Courtesy of Aleksander Tavgen (@atavgen)
The risk-management loop — a tech-driven approach to patient data and safety monitoring
We now describe a tech-driven approach for monitoring data and safety in tech-driven clinical trials. We call this the risk-management loop.

Traditional study monitoring is like driving up the parkway. No rest-stops for the next 25 miles.
If you are driving up Route 17 in New Jersey and you miss the exit to Ikea, you drive15 miles north and come back. You will probably get stuck in traffic.
In a loop — you just drive around a second time and exit to Ikea.
A risk management loop — faster and more agile
A risk management loop provides a systematic and tech-driven way to think about decentralized clinical trial risk. The tech-driven approach is agile, and acknowledges the fact that decentralized clinical trials are technology-intensive and dependent upon reliability of complex software systems which are vulnerable in many different ways.
The clinical trial risk management loop
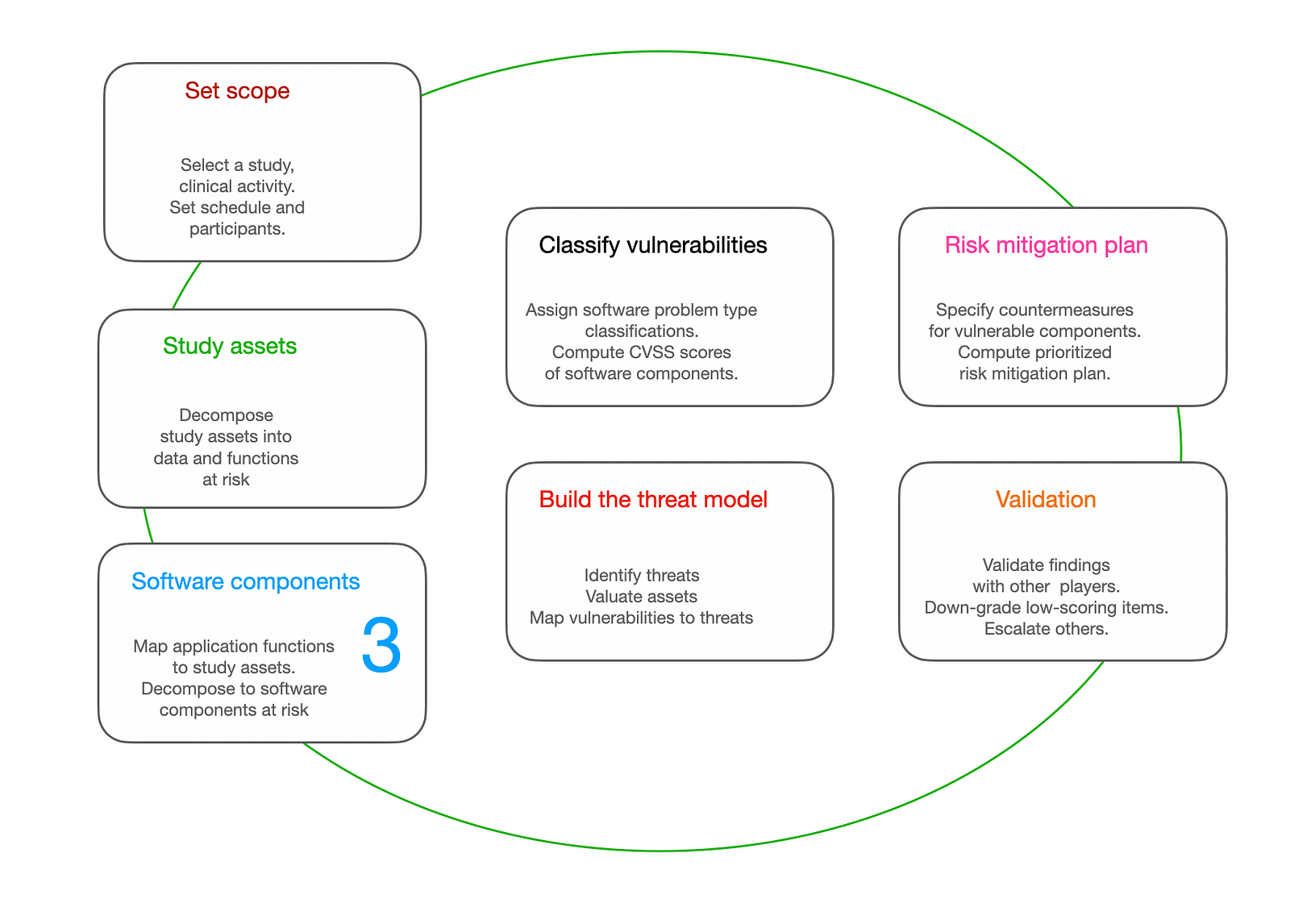
You can start anywhere and circle back, fixing assumptions you had and injecting new observations as the decentralized clinical trial progresses.
The decentralized clinical trial risk analysis and risk management loop has 7 steps as described in the above schematic. The steps of the loop use threat modeling and quantitative risk assessment methods based on providing a financial value to assets (such as devices, sites and patient records) in order to determine value at risk and prioritize risk countermeasures.
Summary
Outdated data and safety monitoring processes have fallen behind the volume, velocity and variety of data in DCT.
The risk-management loop approach acknowledges the fact that there will be changes to the clinical trial protocol, changes in regulation, changes in environment (COVID-19, chip shortages, supply-chain delays) and unexpected events in drug safety and efficacy.
This post was originally posted on Danny Lieberman’s Medium account.
Danny Lieberman is a solid-state physicist by training, serious amateur musician and tech entrepreneur. Involved developing AI-driven automation of data integrity and patient safety monitoring for clinical trials. He is proud to be working with some very smart people, all smarter than him.





