SDV sucks
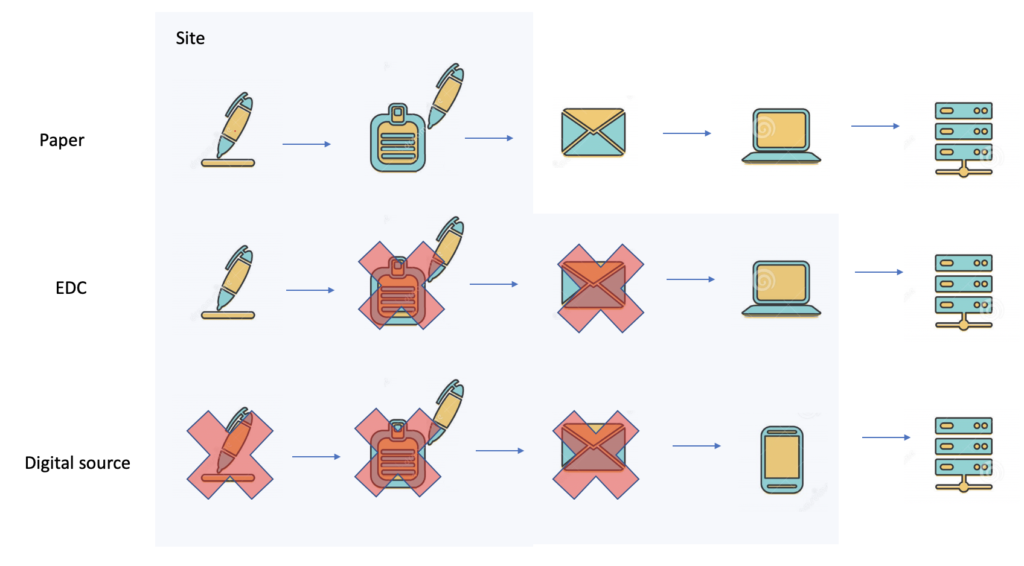
How professional services delay cures for patients. Why SDV Sucks In the early 50s of the previous century, nobody knew that 70 years later clinical trials could be run using a phone. Clinical trials that were performed 70 years ago used paper forms. By the late 70s, batch-driven data processing systems were introduced. Paper forms […]
Career development for clinical data managers

A good clinical data manager is an essential piece of running a clinical trial. A well-trained and responsibility clinical data manager is a an important part in study execution. A good clinical data manager will want to have a career development plan. A clinical data manager on an individual contributor track, needs to develop […]
Temperature excursions and APIs to reduce study monitor work
I did a lot of local excursions the past 3 days – Jerusalem, Tel Aviv, Herzliya and Haifa. For some reason, the conversations with 2 prospects had to do with refrigerators. I do not know if this is Freudian or not, considering the hot weather of July in Israel. The conversations about refrigerators had to […]
Urban medical legends
Because I was trained as a solid-state physicist I am skeptical of many medical claims – including the efficacy of digital health apps. Gina Kolata wrote this post last week. I’ll let you decide for yourself. You might assume that standard medical advice was supported by mounds of scientific research. But researchers recently discovered that […]
What takes precedence? GCP or hospital network security?
This is a piece I wrote a while back on my medical device security blog – Cybersecurity for medical devices. One of the biggest challenge of using connected medical devices in clinical trials is near real-world usage of devices that are not commercially-ready. We have a couple of customers that are performing clinical trials of […]
How to measure clinical response in medical device clinical trials
It is 19:15 and daylight savings time. It is too hot to go out and run or bike. Time to write. Today we were helping a customer with hardware issues. At the end of a long day, I started thinking that even hardware issues are valuable data to the decision-making process of measuring efficacy of […]
Bionic M2M: Are Skin-mounted M2M devices – the future of clinical trials?
There is a lot of hype about wearables. One of the best ways to correlate patient compliance with patient biometrics is for the patient to wear the sensor on her skin. I started thinking about skin-mounted devices again after reading the press release about the BioSerenity Series B, so I dug up an essay I wrote […]
Living off generic solutions developed in the past
I recently read some posts on Fred Wilson’s blog and it was impressive that he writes every day. I’ve fallen into the trap of collecting raw material and then waiting to find time to write a 2000-word essay on some topic of importance to me. But, I think it was Steve Jobs who said the […]
Killed by code – back to the future
I hope that the code in your digital therapeutic for treating autistic children, doesn’t look like this. Back in 2011, I thought it would only be a question of time before we have a drive by execution of a politician with an ICD (implanted cardiac device). In Jan 9, 2017 FDA reported in a FDA Safety […]
Teetering on the precipice of medical device/digital health clinical trials
Danny teeters on the edge of the precipice of privacy and security. Step on the brakes not on the gas and don’t look down. Take a 500m leap of faith into the chasm of medical device clinical trials. Validate digital therapeutics. Venture into uncharted territory of medical cannabis trials. At some stage in my “let’s […]
