How to meet the 10 top challenges in Phase 1 clinical trials

Phase 1 challenges are unlike larger Phase 2, Phase 3 studies. The science is still unsure. The the clinical operations team at a startup may still be under construction. In this post, I’ll share our experiences at flaskdata.io with early stage drug and device vendors doing their first Phase 1 safety study. You’ll see a […]
SDV sucks
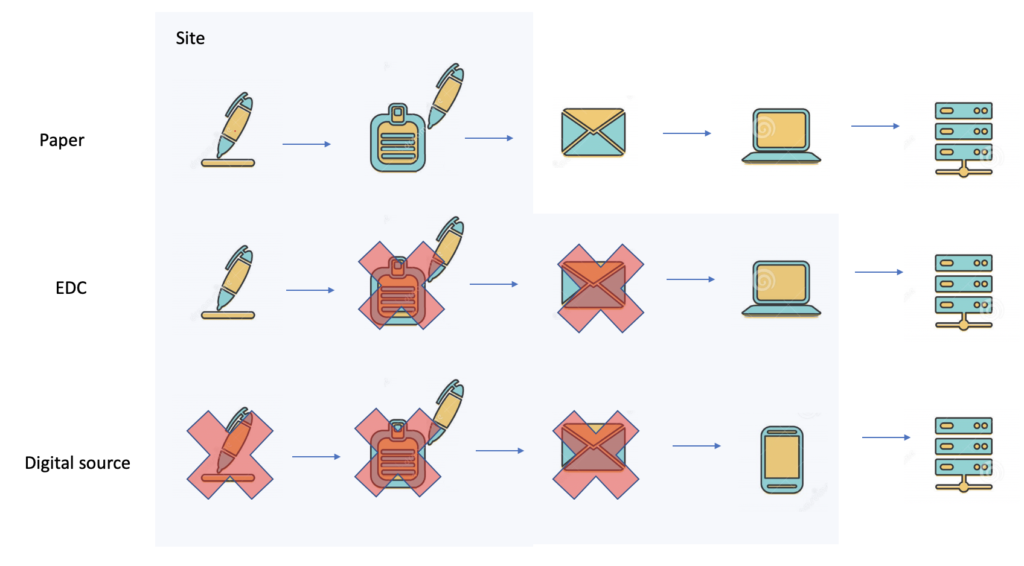
How professional services delay cures for patients. Why SDV Sucks In the early 50s of the previous century, nobody knew that 70 years later clinical trials could be run using a phone. Clinical trials that were performed 70 years ago used paper forms. By the late 70s, batch-driven data processing systems were introduced. Paper forms […]
Career development for clinical data managers

A good clinical data manager is an essential piece of running a clinical trial. A well-trained and responsibility clinical data manager is a an important part in study execution. A good clinical data manager will want to have a career development plan. A clinical data manager on an individual contributor track, needs to develop […]
3 ways that assure protocol compliance and data integrity in your clinical trials

In this short essay, I’ll take a closer look at how the clinical trial supply chain is evolving. There is now rapid change from traditional site-centric trials to patient-centric operations that assure data integrity and safety monitoring We’ll see how the pandemic, innovation in clinical operations models and consumer technology drive the change to patient-centric research. […]
The LA Freeway model of clinical monitoring
A freeway paradigm helps explain why onsite visits by study monitors don’t work and helps us plan and implement an effective system for protocol compliance monitoring of all sites, all data, all the time that saves time and money. But first – let’s consider some special aspects of clinical trial data: Clinical trial data is highly dimensional data. Clinical […]
10 ways to detect people who are a threat to your clinical trial
Flaskdata.io helps Life Science CxO teams outcompete using continuous data feeds from patients, devices and investigators mixed with a slice of patient compliance automation. One of the great things about working with Israeli medical device vendors is the level of innovation, drive and abundance of smart people. It’s why we get up in the morning. […]
Urban medical legends
Because I was trained as a solid-state physicist I am skeptical of many medical claims – including the efficacy of digital health apps. Gina Kolata wrote this post last week. I’ll let you decide for yourself. You might assume that standard medical advice was supported by mounds of scientific research. But researchers recently discovered that […]
How to measure clinical response in medical device clinical trials
It is 19:15 and daylight savings time. It is too hot to go out and run or bike. Time to write. Today we were helping a customer with hardware issues. At the end of a long day, I started thinking that even hardware issues are valuable data to the decision-making process of measuring efficacy of […]
The gap between the proletariat and Medidata (or should I say Dassault)
We need a better UX before [TLA] integration The sheer number and variety of eClinical software companies and buzzwords confuses me. There is EDC, CTMS, IWRS, IVRS, IWRS, IRT, eSource, eCOA, ePRO and a bunch of more TLAs. For the life of me I do not understand the difference between eCOA and ePRO and why […]
How to annoy your eClinical platform vendor
Every question is a cry to understand the world. There is no such thing as a dumb question. Carl Sagan In this guest post, my colleague Tigran Arzumanov asks questions about questions. Tigran is an experienced and highly talented business developer for life science companies and he’s been around the block a few times. What […]
