How to ensure patient adherence in decentralized clinical trials

Patient adherence in clinical trials without technology How can you assure patient adherence to the protocol, when you don’t see the patient? Life science companies are increasingly turning to decentralized clinical trial models. This means that patients are home alone. They use ePRO to record side effects and outcomes from the treatment. With 10X more […]
SDV sucks
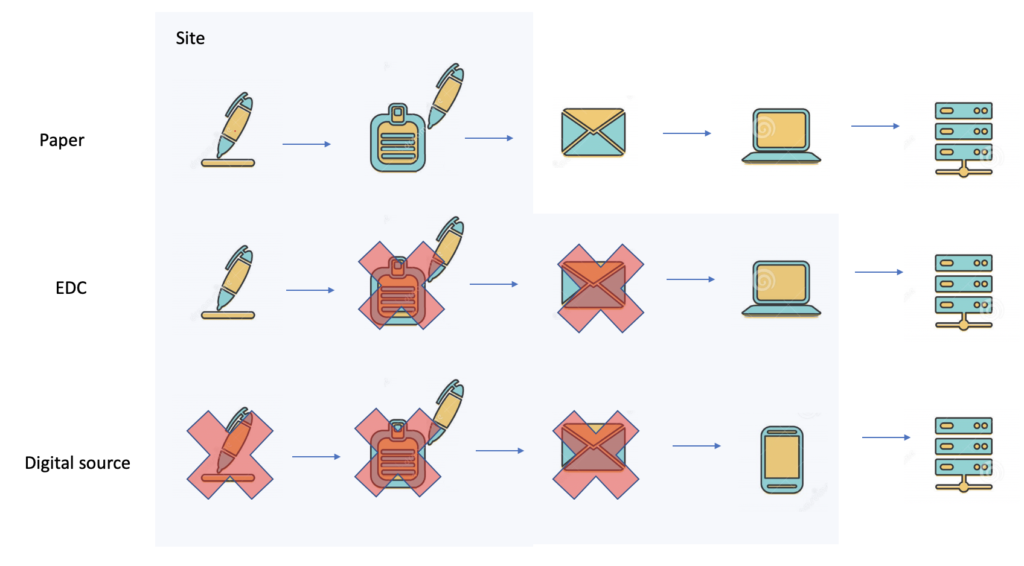
How professional services delay cures for patients. Why SDV Sucks In the early 50s of the previous century, nobody knew that 70 years later clinical trials could be run using a phone. Clinical trials that were performed 70 years ago used paper forms. By the late 70s, batch-driven data processing systems were introduced. Paper forms […]
GCP for clinical trials – patterns of low-concern and high-impact

How to assure GCP for clinical trials in the best way? In this post, we will show you how to assure good clinical practice in clinical trials. According to Cancer.gov, GCP (Good Clinical Practice) is an international set of guidelines that helps make sure that the results of a clinical trial are reliable and that […]
Data capture by sites is activity, not achievement

Never mistake data collection activity for an achievement Recruiting and caring for patients (whether at home or on site) is a research site responsibility. Capturing pages (note the paper paradigm!) is one of the key billable metrics for a site. While data collection, detection of exceptions and action to close issues are all activities essential […]
Living in an ideal world where the site coordinator is not overwhelmed by IT

Tigran examines the idea of using EDC edit checks to assure patient compliance to the protocol. How should I assure patient compliance to the protocol in my clinical trials? I get asked sometimes whether automated patient compliance deviation detection and response is not overkill. After all, all EDC systems allow comparing input to preset ranges and […]
Using automated detection and response technology mitigate the next Corona pandemic
What happens the day after? What happens next winter? Sure – we must find effective treatment and vaccines. Sure – we need to reduce or eliminate the need for on-site monitoring visits to hospitals in clinical trials. And sure – we need to enable patient monitoring at home. But let’s not be distracted from 3 […]
Temperature excursions and APIs to reduce study monitor work
I did a lot of local excursions the past 3 days – Jerusalem, Tel Aviv, Herzliya and Haifa. For some reason, the conversations with 2 prospects had to do with refrigerators. I do not know if this is Freudian or not, considering the hot weather of July in Israel. The conversations about refrigerators had to […]
Doctor-Patient Communication – the key to success and the struggle to succeed.
Katherine Murphy, Chief Executive of the Patients Association London once said, “The huge rise in complaints in relation to communication and a lack of respect are of particular concern. Patients are not receiving the compassion, dignity and respect which they deserve.” As clinical trial technology guys, you would assume that we love code more than […]
Urban medical legends
Because I was trained as a solid-state physicist I am skeptical of many medical claims – including the efficacy of digital health apps. Gina Kolata wrote this post last week. I’ll let you decide for yourself. You might assume that standard medical advice was supported by mounds of scientific research. But researchers recently discovered that […]
Why Microsoft is evil for medical devices
Another hot day in paradise. Sunny and 34C. Not a disaster but still a PITA We just spent 2 days bug-fixing and regression-testing code that was broken by Microsoft’s June security update to Windows operating systems and Explorer 11. Most of the customers of the FlaskData EDC, ePRO, eSource and automated detection and response […]
