SDV sucks
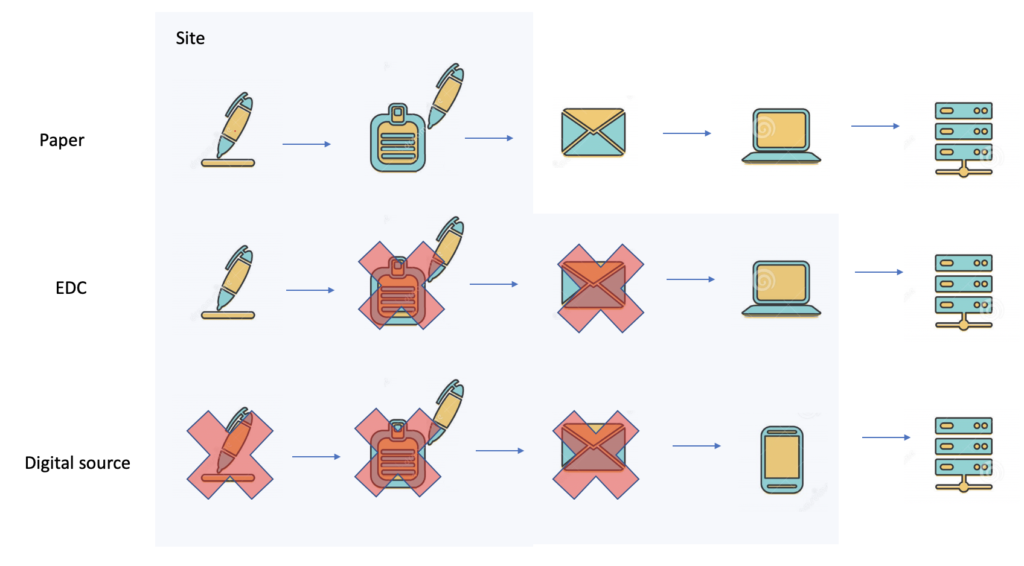
How professional services delay cures for patients. Why SDV Sucks In the early 50s of the previous century, nobody knew that 70 years later clinical trials could be run using a phone. Clinical trials that were performed 70 years ago used paper forms. By the late 70s, batch-driven data processing systems were introduced. Paper forms […]
Clinical data management, payment for outcomes

Payment for outcomes using GCP metrics is a way to significantly reduce clinical operations costs and increase value-for-money from your CRO or DCT vendor. Payment for outcomes using GCP metrics shifts study costs from billable activities to measurable results. Professional service activities are hard to control. GCP metrics are hard to contest. In this post, […]
GCP for clinical trials – patterns of low-concern and high-impact

How to assure GCP for clinical trials in the best way? In this post, we will show you how to assure good clinical practice in clinical trials. According to Cancer.gov, GCP (Good Clinical Practice) is an international set of guidelines that helps make sure that the results of a clinical trial are reliable and that […]
Why you do not want to unify data in your clinical trial

Unified clinical data sets – good or bad? Unification of data from patient medical records, hospital reports and clinical trial protocols is a tempting yet extremely dangerous idea. In this outstanding guest post, security and privacy expert, Veronika Valdova from Arete-Zoe explains why merging medical records, hospital reports, and clinical trial data is a very bad idea. How data breaches […]
Living in an ideal world where the site coordinator is not overwhelmed by IT

Tigran examines the idea of using EDC edit checks to assure patient compliance to the protocol. How should I assure patient compliance to the protocol in my clinical trials? I get asked sometimes whether automated patient compliance deviation detection and response is not overkill. After all, all EDC systems allow comparing input to preset ranges and […]
3 ways that assure protocol compliance and data integrity in your clinical trials

In this short essay, I’ll take a closer look at how the clinical trial supply chain is evolving. There is now rapid change from traditional site-centric trials to patient-centric operations that assure data integrity and safety monitoring We’ll see how the pandemic, innovation in clinical operations models and consumer technology drive the change to patient-centric research. […]
The LA Freeway model of clinical monitoring
A freeway paradigm helps explain why onsite visits by study monitors don’t work and helps us plan and implement an effective system for protocol compliance monitoring of all sites, all data, all the time that saves time and money. But first – let’s consider some special aspects of clinical trial data: Clinical trial data is highly dimensional data. Clinical […]
So what’s wrong with 1990s EDC systems?
Make no doubt about it, the EDC systems of 2020 are using a 1990’s design. (OK – granted, there are some innovators out there like ClinPal with their patient-centric trial approach but the vast majority of today’s EDC systems, from Omnicomm to Oracle to Medidata to Medrio are using a 1990’s design. Even the West […]
I love being a CRA, but the role as it exists today is obsolete.
I think that COVID-19 will be the death knell for on-site monitoring visits and SDV. Predictions for 2020 and the next generation of clinical research – mobile EDC for sites, patients and device integration that just works. I’m neither a clinical quality nor a management consultant. I cannot tell a CRO not to bill […]
Using automated detection and response technology mitigate the next Corona pandemic
What happens the day after? What happens next winter? Sure – we must find effective treatment and vaccines. Sure – we need to reduce or eliminate the need for on-site monitoring visits to hospitals in clinical trials. And sure – we need to enable patient monitoring at home. But let’s not be distracted from 3 […]
